Excited molecular orbital n2 state ground dinitrogen cation so diagram configuration molecule theory electron explain sigma states why chemistry mathrm Orbital molecular paramagnetic oxygen theory bond chemistry energy molecule o2 bonding level electron diagrams electrons unpaired predicts answer valence libretexts Orbital molecular molecules diagram orbitals diatomic bonding of2 delocalized bond atomic libretexts electrons chem correlation hybridization atoms np homonuclear pageindex
Chemical Forums: Molecular Orbital Diagram
Chemical forums: molecular orbital diagram Orbital orbitals mo prepare molecule interact atomic represented Diagram n2 mo orbital molecular diagrams electrons chemistry electron lie together two explain determined improve answer stack sponsored links via
Physical chemistry
Orbital molecular diagram cl2 s2 molecule bond orbitals electron unpaired bonding c2 energy theory valence electrons paramagnetic molecules diatomic atom9.10: molecular orbital theory predicts that molecular oxygen is Molecular orbital theoryDelocalized bonding and molecular orbitals.
Orbitals bonding electrons valence orbital energy chemistry delocalized libretexts ion chemChemistry molecular orbitals orbital diagram energy bonding level edu wave two function h2 theory bond atomic molecule chemwiki atoms atom Orbital molecular theoryMolecular orbital theory.

Solved: chapter 5 problem 7p solution
Chapter 6.5 delocalized bonding and molecular orbitalsOrbital molecular diagrams molecules origins chemistry mathematics gif does electrons numbers Understanding molecular orbital theory.
.


physical chemistry - How can two electrons lie together in an orbital

9.10: Molecular Orbital Theory Predicts that Molecular Oxygen is

molecular orbital theory - How to explain the excited states in the

Chemical Forums: Molecular Orbital Diagram

Molecular Orbital Theory - Chemistry LibreTexts

mathematics - Origins of molecular orbital diagrams? - History of
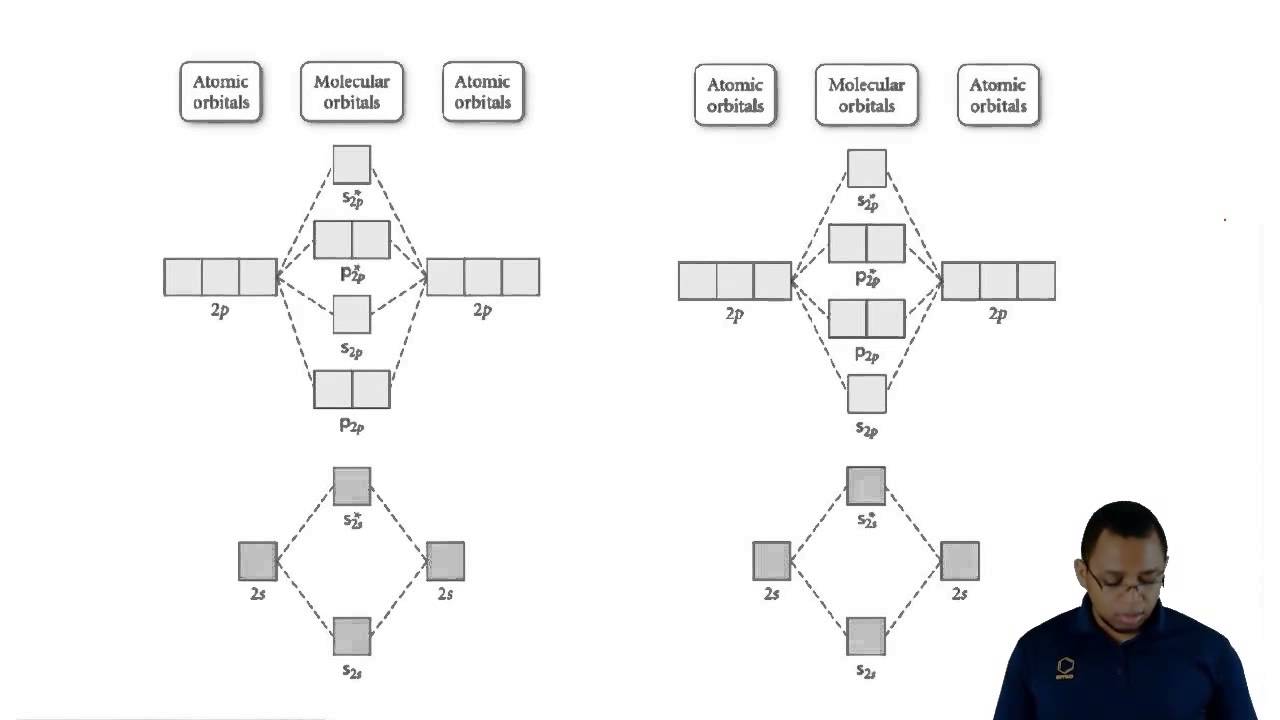
Understanding Molecular Orbital Theory - YouTube

Chapter 6.5 Delocalized Bonding and Molecular Orbitals - Chemistry